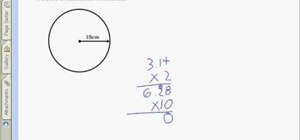
To calculate percentage composition we will take the example CO2 it stands for Carbon-Di-Oxide. It is the composition of Carbon and Oxygen. We have one carbon and its weight is 12.01 and 2 oxygen. So, we will multiply 2 with 16.00 which is the weight of the oxygen.
C - 1* 12.01=12.01
O - 2* 16.00=32.00
-------
44.01 g CO2
This is the total weight of CO2. Now, to know the percent composition of Carbon and Oxygen .We will divide their weight with the total weight of CO2.
% C = 12.01/44.01 = 0.2729= 27.29% (we will divide 0.2729 with 100 to take the percentage).
So , % C in CO2 is 27.29%
% O = 32.00/44.01 =0.7271 =72.71 %O in CO2.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.



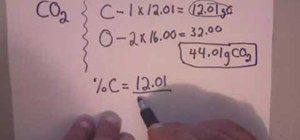














Be the First to Comment
Share Your Thoughts