If you want to know how to calculate percent error easily, you should watch this video. Error is the amount of deviation from accurate values. Error calculation is not possible, unless you make a quantitative measurement of the various quantities involved in your experiment. Measurement can help us in calculating errors and knowing how right we are in our theoretical models. In calculating percent error, We need to get the experimental value and the value which you are aiming at, which is the actual value first. Subtract the experimental value from the actual value and take its absolute value. Divide the raw error by the actual value and multiply the relative error by 100 to get the percent error. You're done.
Apple's iOS 26 and iPadOS 26 updates are packed with new features, and you can try them before almost everyone else. First, check Gadget Hacks' list of supported iPhone and iPad models, then follow the step-by-step guide to install the iOS/iPadOS 26 beta — no paid developer account required.















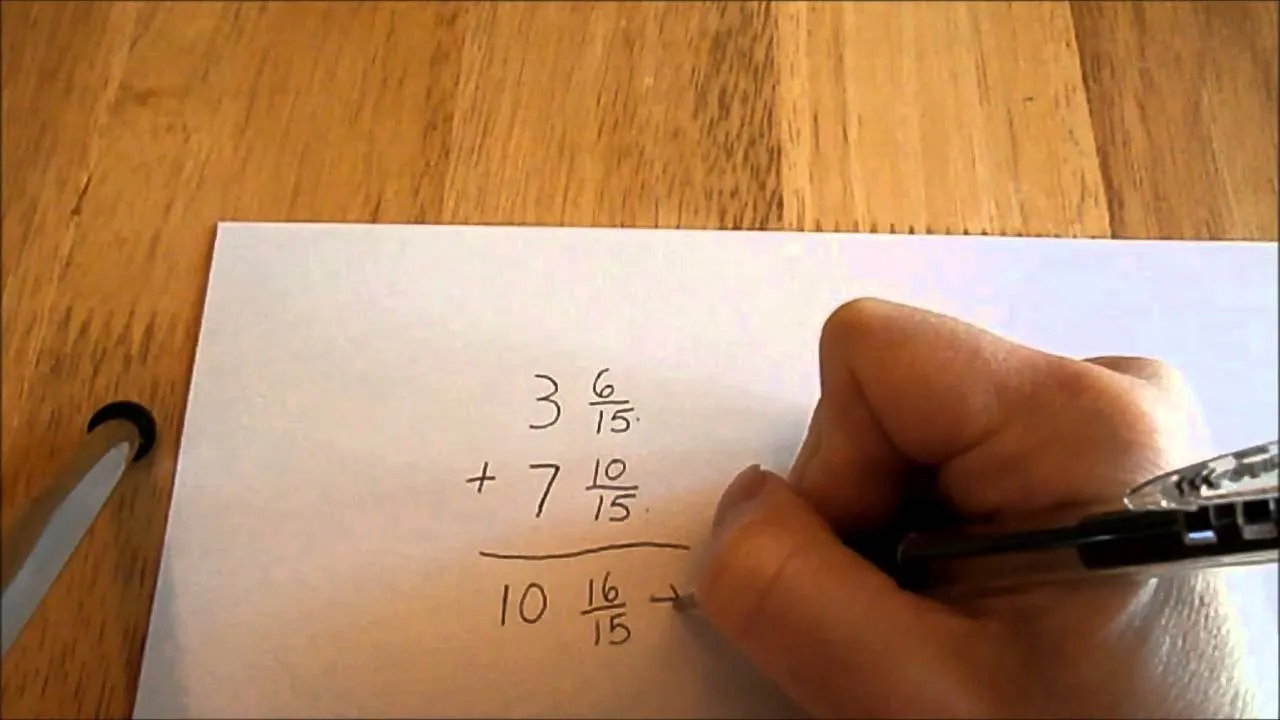

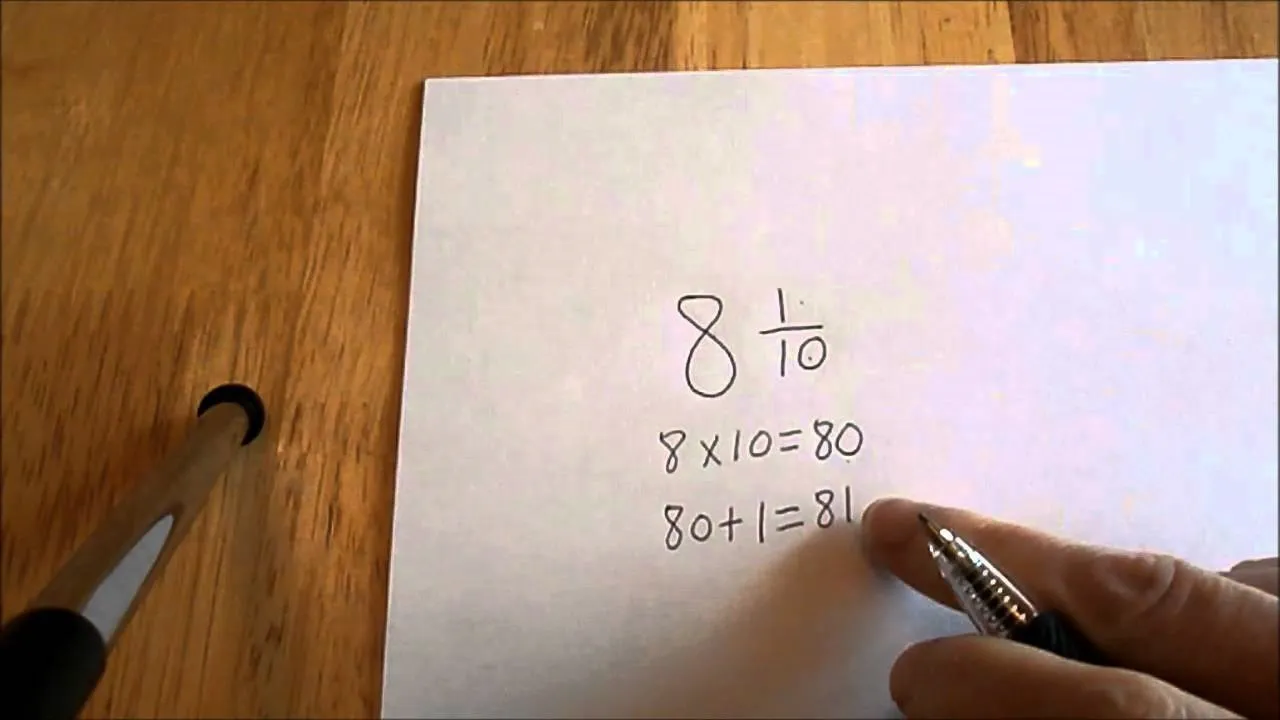


Comments
Be the first, drop a comment!