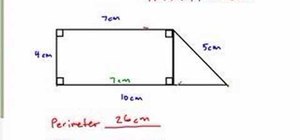
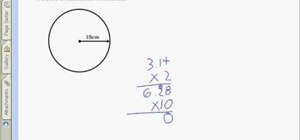
If you want to know how to calculate percent error easily, you should watch this video. Error is the amount of deviation from accurate values. Error calculation is not possible, unless you make a quantitative measurement of the various quantities involved in your experiment. Measurement can help us in calculating errors and knowing how right we are in our theoretical models. In calculating percent error, We need to get the experimental value and the value which you are aiming at, which is the actual value first. Subtract the experimental value from the actual value and take its absolute value. Divide the raw error by the actual value and multiply the relative error by 100 to get the percent error. You're done.
Just updated your iPhone? You'll find new emoji, enhanced security, podcast transcripts, Apple Cash virtual numbers, and other useful features. There are even new additions hidden within Safari. Find out what's new and changed on your iPhone with the iOS 17.4 update.







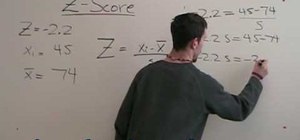




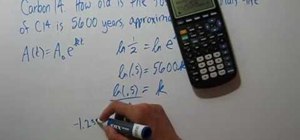






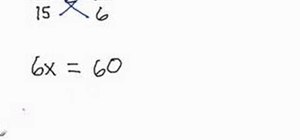
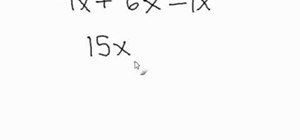
1 Comment
I do not believe this to be accurate; it is a very common mistake. To begin with, error is calculated as: experimental value - actual value. The formula is set up so larger values are positive and lesser values are negative. Then divide the absolute value of the error by the absolute value of the actual value and multiply be 100 to get the percent. To demonstrate the importance of using the absolute value of the denominator, take the case of an actual value of -4 and an experimental value of -3. Plugging into your formula would give a percent error of -25% when in reality it is 25%.
Share Your Thoughts