In chemistry you come across problems which ask you to find the percent by mass and percent composition of each element in a chemical compound. To do this the first step is to compute the molar mass. Use the periodic table to look up the mass of individual atoms and multiply it by the number of atoms to find the mass of that element. Now add up all the masses to arrive at the molar mass of the compound. Now to find the percent composition of an element divide the total mass of each element by the molar mass and multiply by 100. In this way compute the percent mass of each element. Finally if you add up all these percentages the total should be approximately equal to 100 in case you rounded any values. This video shows how to find the percent mass of each elements in a chemical compound.
Apple's iOS 26 and iPadOS 26 updates are packed with new features, and you can try them before almost everyone else. First, check Gadget Hacks' list of supported iPhone and iPad models, then follow the step-by-step guide to install the iOS/iPadOS 26 beta — no paid developer account required.




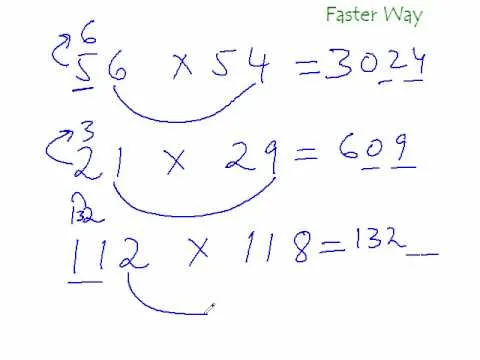
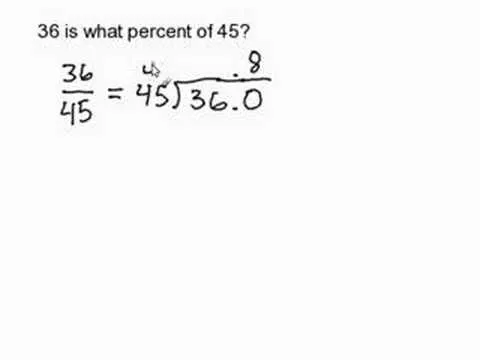
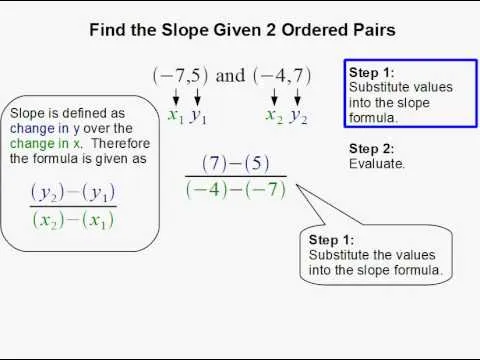











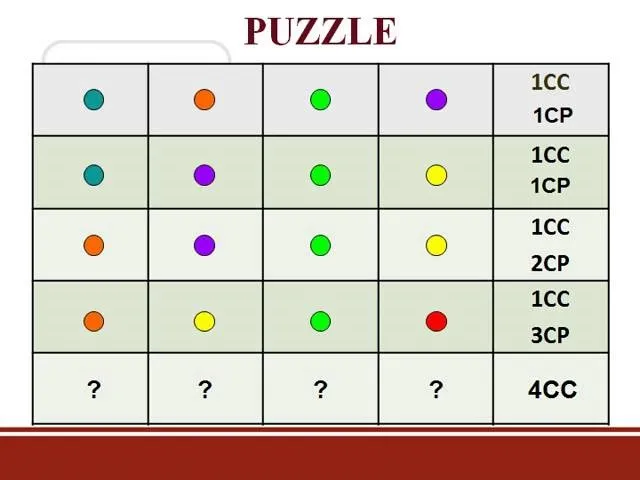



Comments
Be the first, drop a comment!