The way to convert 0.300 Moles of water into Grams is to start by writing what you have. Then put grams on top and moles on the bottom, then put one by the mole, the omler mass goes by grams. Now you figure out the moler mass of water which has 2 H and 1 O, its 2 times 1 which is the atomic mass for hydrogen plus 16.0 for oxygen which gives a total of 18.0 g/mol. So then you insert the 18 up by the grams, now you can cancel out the moles and multiply 0.300 times 18.0g and it gives you 5.40 g, and the substance is water so the answer is 5.40g H20
Apple's iOS 26 and iPadOS 26 updates are packed with new features, and you can try them before almost everyone else. First, check Gadget Hacks' list of supported iPhone and iPad models, then follow the step-by-step guide to install the iOS/iPadOS 26 beta — no paid developer account required.




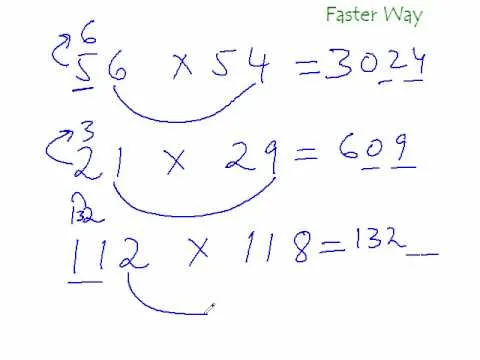
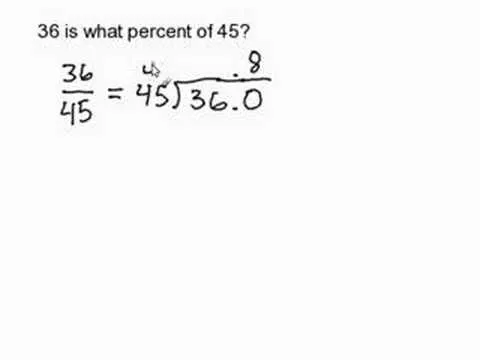
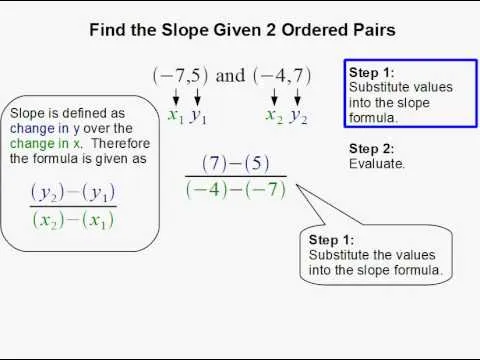











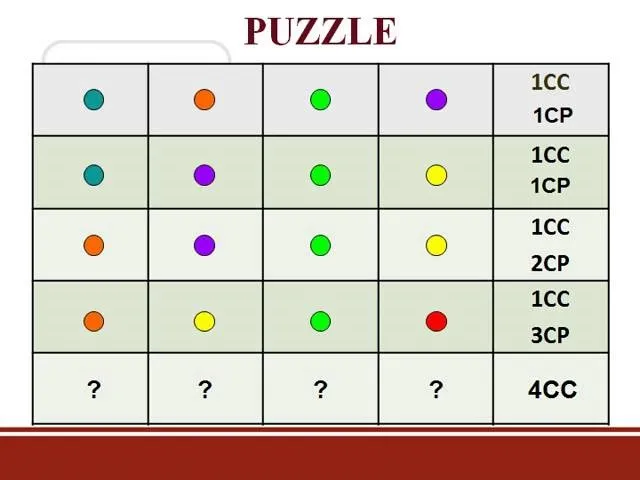
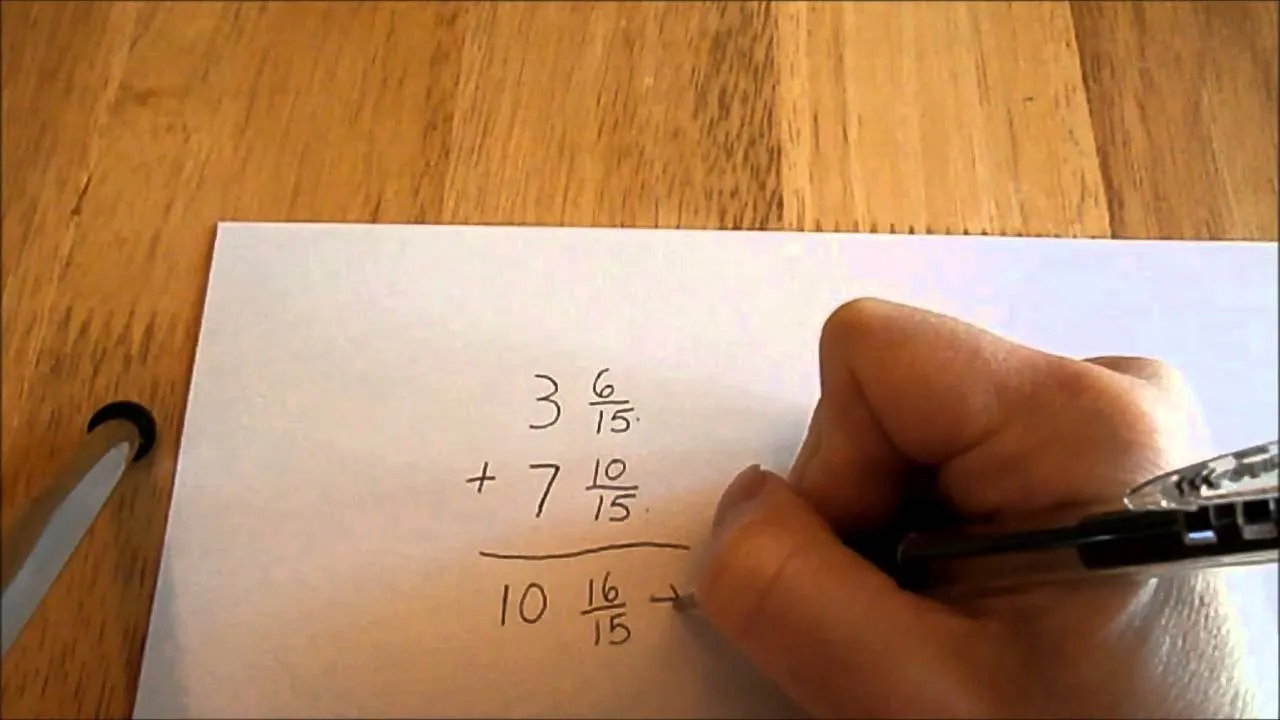

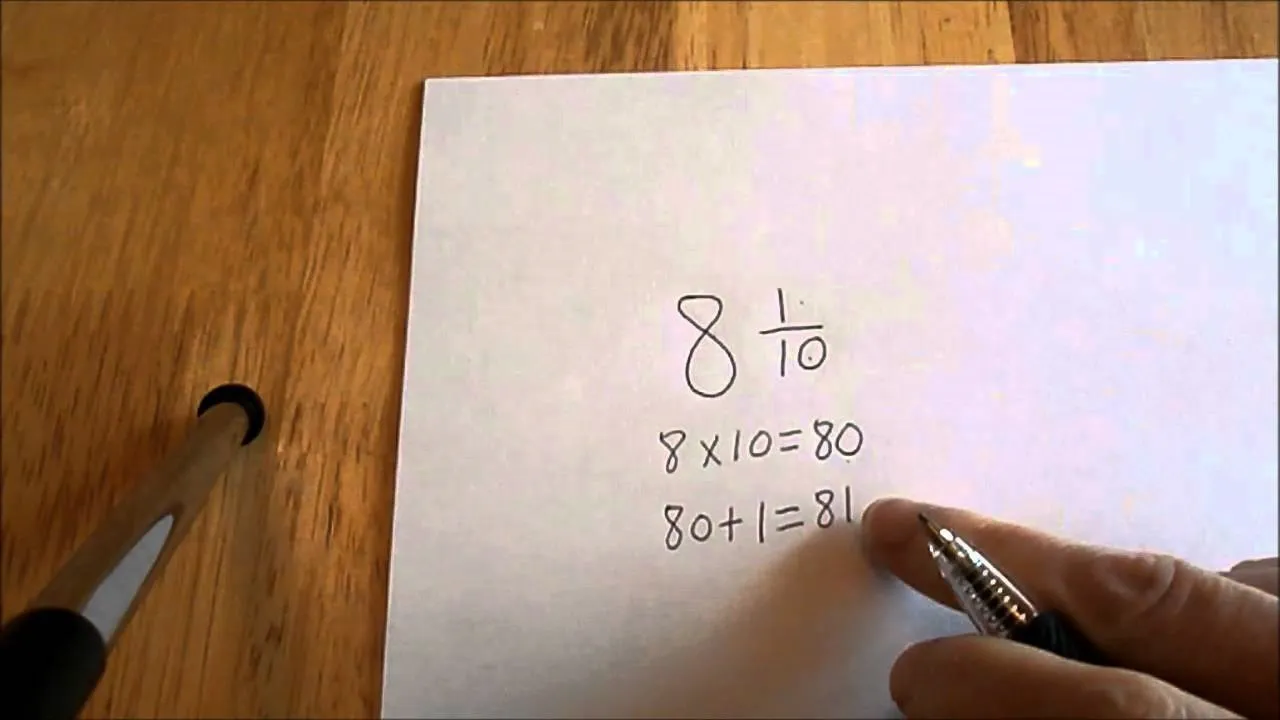


Comments
Be the first, drop a comment!